Water softener is required for the majority if water treatment plans, and when using water softener you also require a brine tank full of salt. But what is this for and why is it needed? Let’s find out.
Water softener is required for the majority if water treatment plans, and when using water softener you also require a brine tank full of salt. But what is this for and why is it needed? Let’s find out.
Water hardness is the concept used to describe the mineral content of water. Water which contains a relatively large number of minerals (calcium and magnesium) is described as hard, while water with low mineral content is described as soft.
One of the most common ways to soften hard water is through the use of salt. Most people and water treatment companies will use an ion-exchange water softener, where salt plays a critical role in the functionality of these water softening systems.
Ion Exchange is the process of replacing a molecule of lesser charge for a charged molecule. The ion exchange uses salt ions to replace calcium and magnesium in the water supply.
Lets break it down.
Ion-exchange water softeners have two or more tanks; One softener tank contains the resin beads, while the other softener tank contains a salty brine solution.
How it works
1. Hard water enters the water softener via the Resin Tank (ion-exchange system) and flows through resin beads. The resin beads in the ion-exchange system are positively charged and are coated with negatively charged sodium ions.
 The minerals contained in hard water are negatively charged. When the hard water passes through the resin beads, the minerals contained in the water are pulled away from the water and towards the resin beads.
The minerals contained in hard water are negatively charged. When the hard water passes through the resin beads, the minerals contained in the water are pulled away from the water and towards the resin beads.
At the same time, the sodium ions which are attached to the resin beads are attracted over to the water molecule. This exchange of mineral ions for sodium ions allows the water molecule to maintain a balanced charge.
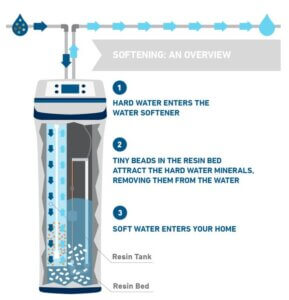
Essentially, calcium and magnesium ions are captured by the media inside and removed from the water before it travels to the rest of your system.
2. This ion exchange results in soft water that enters your facility.
Now we are left with the hard minerals which were in the water, it needs to be recharged, and that’s when the softener salts go to work.
3. To regenerate the system the resin tank is flushed with salt water from the brine tank.
The salt mixes with water to create a brine solution, this solution flows through the resin, contacting the resin beads loaded with calcium and magnesium ions.
The sodium in the brine solution has a positive charge, and breaks away to the negatively charged resin. When that happens, the calcium and magnesium molecules get kicked off the resin until all exchange sites are taken up by sodium ions.
4. The minerals and brine solution are then flushed from the system and down the drain, and the system is ready to begin the softening water treatment again.

To clarify water softeners do not add any salt to the water; the water softening process breaks down the salt to use its sodium only.
How Much Sodium is in Your Water?
The amount of sodium in softened water depends on how hard the water is to begin with. The ion exchange process is just that, an exchange, the harder the water, the more sodium is required to remove the calcium and magnesium.
Final Thoughts
Salt is critical for water softening systems that use ion-exchange, these systems remove the minerals in hard water and replace them with sodium ions.
It all comes down to chemistry; that’s where our experts come in! contact or team to day to talk about all of your water softener needs.