Water alkalinity and pH are similar, but they are not the same. Water pH measures the amount of hydrogen (acid ions) in the water, whereas water alkalinity is a measure of the carbonate and bicarbonate levels in water
When thinking about water quality, alkalinity is more important than pH. pH tells you whether the water is acidic, neutral or basic, but not the buffering capacity of the water.
Buffering capacity is the ability of water (or compound) to resist a change in pH. Alkalinity tells you the buffering capacity in the basic pH range of the water.
About pH
pH stands for Potential Hydrogen, and in order to control alkalinity, you have to control the pH.
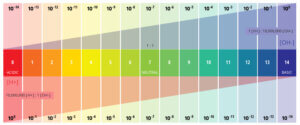
pH is a numeric scale used to specify the acidity, the scale runs from 0 to 14 and if it is located lower on the scale, it is more acidic. If it is located higher on the scale, it is more basic, or alkaline. Processed foods and products like yogurt, fish, and cheese are typically more acidic while vegetables like beets, bell peppers, and kale are high alkaline.
Acidic water (low pH) can leach metals from plumbing systems, which can cause pipes to leak. Metals that leach from the pipes (lead from lead pipes or copper from copper pipes) may also cause health problems.
Water with a value greater than 7 indicates alkalinity and tends to affect the taste of the water. Alkaline drinking water may take on a “soda” taste.
pH levels in drinking water are monitored extremely closely and are required to be kept in a range of 6.5–8.5 by the Environmental Protection Agency (EPA), where 7 indicates the neutral point.
Factors that Influence the pH of Water
There are many factors that can affect pH in water, both natural and man-made. Most natural changes occur due to interactions with surrounding rock (particularly carbonate forms) and other materials. pH can also fluctuate with precipitation (especially acid rain) and wastewater or mining discharges.
Carbon Dioxide
Carbon dioxide is the most common cause of acidity in water.
Photosynthesis, respiration and decomposition all contribute to pH fluctuations due to their influences on CO2 levels. The extremity of these changes depends on the alkalinity of the water, but there are often noticeable diurnal (daily) variations. This influence is more measurable in bodies of water with high rates of respiration and decomposition.
Natural pH Influences
Carbonate materials and limestone are two elements that can buffer pH changes in water. Calcium carbonate (CaCO3) and other bicarbonates can combine with both hydrogen or hydroxyl ions to neutralize pH. When carbonate minerals are present in the soil, the buffering capacity (alkalinity) of water is increased, keeping the pH of water close to neutral even when acids or bases are added. Additional carbonate materials beyond this can make neutral water slightly basic.
Man-Made pH Influencers
Pollution in the air, soil or directly in the levels of CO2 can also further decrease the pH of rain.
Point source pollution is a common cause that can increase or decrease pH depending on the chemicals involved ¹⁸. These chemicals can come from agricultural runoff, wastewater discharge or industrial runoff. Mining operations (particularly coal) produce acid runoff and acidic groundwater seepage if the surrounding soil is poorly buffered ²². Wastewater discharge that contains detergents and soap-based products can cause a water source to become too basic.
What is Alkalinity?
While alkalinity and pH are closely related, there are distinct differences. While pH indicates if a solution is an acid or a base, alkalinity tells you how much acid a solution can absorb without changing the pH.
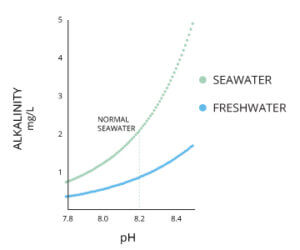
It is essentially, the buffering capacity of a solution (aka water). Therefore, solutions with low alkalinity have a lower buffering capacity, and change pH rather quickly when something acidic is added. Inversely, high alkalinity samples have a higher buffering capacity and are less affected when something acidic is added; you have to add a lot more acid in order to obtain the same pH change as in a low alkalinity sample.
Without this acid neutralizing capacity, any acid added to a river would cause an immediate change in the pH.
Why Measure Alkalinity?
Measuring alkalinity is important to determining waters ability to neutralize acidic pollution (as measured by pH) from rainfall or snowmelt.
Alkalinity is important in a variety of industries, everything from aquariums and aquaculture to plating and water treatment requires alkalinity testing. Not knowing the alkalinity of the water in a multiple of fields can have disastrous implications and results for the final product. Correctly monitoring alkalinity can save users and operators time, materials, and money.
The alkalinity of water also plays an important role in daily pH levels, the process of photosynthesis by algae and plants uses hydrogen, thus increasing pH levels. Likewise, respiration and decomposition can lower pH levels. Most bodies of water are able to buffer these changes due to their alkalinity, so small or localized fluctuations are quickly modified and may be difficult to detect.
For more information don’t hesitate to get in touch with us.







